作者简介:杨晓,女,1988年生,学士,主要从事miRNA和肿瘤的相关研究。
共同通讯作者:孟庆贺,联系电话:0577-86689805;吕建新,联系电话:0577-86689805。
观察前列腺癌PC-3细胞中微小RNA(microRNA,miRNA)-221和miRNA-222对细胞增殖和迁移的作用,以及对沉默信息调节因子1(SIRT1)表达的影响。
方法设立无处理细胞组(简称control组)、转染空载质粒pEX-5组(简称pEX-5组)、转染miRNA-221 反义抑制质粒组(简称miRNA-221抑制组)、转染miRNA-222 反义抑制质粒组(简称miRNA-222抑制组),应用细胞计数试剂盒(CCK-8法)检测miRNA-221和miRNA-222对前列腺癌细胞增殖的影响;采用细胞划痕修复实验检测miRNA-221和miRNA-222对细胞迁移能力的影响;采用免疫印迹法和荧光定量聚合酶链反应(PCR)检测SIRT1在蛋白水平和mRNA水平的变化;采用miRNAanda靶标进行预测分析。
结果在成功抑制了PC-3细胞中miRNA-221或miRNA-222的表达后,连续7 d检测细胞活性,miRNA-221抑制组和miRNA-222 抑制组的细胞活性(
miRNA-221和miRNA-222能促进前列腺癌PC-3细胞的增殖与迁移能力,影响SIRT1蛋白的表达。
To investigate the roles of micro RNA(miRNA)-221 and miRNA-222 on the proliferation and migration of prostate cancer cell PC-3 and the influence on silent information regulator 1(SIRT1).
MethodsThe groups were arranged as cells without treatment (control group), cells transfected with pEX-5 empty plasmid (pEX-5 group), cells transfected with miRNA-221 inhibitor (miRNA-221 inhibition group) and cells transfected with miRNA-222 inhibitor (miRNA-222 inhibition group). The influence of miRNA-221 and miRNA-222 on prostate cancer cell proliferation was determined by CCK-8, and the migration ability was determined by wound healing test. The protein and mRNA levels of SIRT1 were determined by Western blotting and fluorescence quantitation polymerase chain reaction (PCR), respectively. The prediction analysis was performed by miRNAanda target.
ResultsAfter the expression inhibition of miRNA-221 or miRNA-222 in cell PC-3, the cell activities were determined for 7 d. The activities (
The miRNA-221 and miRNA-222 promote the proliferation and migration of prostate cancer cell PC-3 and have the influence on SIRT1 expression in protein level.
目前,前列腺癌的发病率不断升高,已成为危害老年男性健康的最常见恶性肿瘤之一[ 1, 2]。微小RNA(microRNA,miRNA)在多种肿瘤中表达失调,参与肿瘤发生和发展过程,通过调节细胞的增殖、凋亡、迁移等生物学行为,在肿瘤中发挥癌基因或抑癌基因的作用[ 3, 4]。有研究证实miRNA-221、miRNA-222在包括前列腺癌在内的多种肿瘤中表达上调[ 4, 5, 6]。
沉默信息调节因子(silent information regulator 1,SIRT1)隶属于sirtuin家族,与酵母SIRT2高度同源,为烟酰胺腺嘌呤二核苷酸(NAD+)依赖性的组蛋白去乙酰化酶,参与多种生理过程的调节,包括细胞的增殖、炎症反应等[ 7]。近年来,对SIRT1在癌症发生、发展中的作用也进行了相关研究,但机制尚未统一。SIRT1在癌症的发生、发展中发挥抑癌基因和癌基因双重作用。Wang等[ 8]发现SIRT1在前列腺癌中表达下调,发挥抑癌基因的作用。通过miRNA靶标预测分析,发现miRNA-221、miRNA-222与 SIRT1的3'非转录区(3'untranslated region, 3'UTR)存在靶向结合位点,但尚不清楚miRNA-221、miRNA-222是否通过影响SIRT1的表达而影响前列腺癌细胞的生物学行为。这也是需待解决的一个重要问题。
一、材料
前列腺癌细胞系PC-3购自中国科学院细胞库,用含10% 胎牛血清(美国Gibco公司)的F12培养基(美国Gibco公司)在37 ℃、5%CO2培养箱培养。miRNA-221和miRNA-222干扰质粒pcDNA3.1-hsa-miRNANA-221/222 inhibitor sponge(miRNA-221 inhibitor,miRNA-222 inhibitor)以及空载质粒pEX-5购自上海吉玛生物技术有限公司。转染试剂LipofectamineTM2000 Reagent(美国invitrogen公司);Cell Counting Kit-8(CCK-8)试剂盒(日本同仁公司);兔抗人SIRT1单克隆抗体(1∶2 000稀释,美国Abcam公司)及鼠抗人β-肌动蛋白(β-actin)单克隆抗体(1∶4 000稀释,碧云天公司);辣根过氧化物酶偶联的羊抗兔二抗(1∶2 000稀释,碧云天公司)和羊抗鼠二抗(1∶2 000稀释,碧云天公司);ECL显色液(碧云天公司); 逆转录试剂盒(日本Takara公司); 荧光定量聚合酶链反应(PCR)试剂盒(德国QIAGEN公司)。
二、方法
1.细胞转染 取稳定生长的对数生长期PC-3细胞消化后铺板,根据板的孔径接种不同数目的细胞,96孔板为2 000个/孔,6孔板为3×105个/孔。设立无处理细胞组(简称Control组)、转染空载质粒pEX-5组(简称pEX-5组)、转染miRNA-221 反义抑制质粒组(简称miRNA-221抑制组)、转染miRNA-222 反义抑制质粒组(简称miRNA-222抑制组)。转染前更换为无双抗培养基,按照质粒DNA用量为1 μg/mL、质粒DNA∶LipofectamineTM2000=1∶2的比例转染细胞,6 h后更换新鲜培养基,12~24 h后利用荧光显微镜观察细胞的转染效率。
2.CCK-8法检测细胞增殖 以2×103个细胞/孔铺96孔板,12 h后更换成无双抗培养基,以质粒DNA 0.2 μg /孔,LipofectamineTM2000 0.4 μL/孔分别转染空载质粒pEX-5、miRNA-221 inhibitor、miRNA-222 inhibitor,每组设置5个重复孔,按照CCK-8试剂盒说明书检测细胞的增殖情况( A450 nm值)。
3.划痕实验 取稳定生长的PC-3细胞,消化接种于6孔板,3×105个/孔,第2天更换为无双抗培养基,分别转染pEX-5、miRNA-221 inhibitor、miRNA-222 inhibitor,6 h后更换成新鲜培养基。12 h后用200 μL移液器枪头垂直在6孔板底部画线,磷酸盐缓冲液(phosphate buffered saline,PBS)洗2遍,去掉悬浮细胞,加入1.5%胎牛血清培养基,显微镜拍照,观察0、24、48、72 h的细胞迁移情况。
4.荧光定量PCR检测细胞中miRNA-221、miRNA-222的表达量及SIRT1 mRNA表达的变化 转染48 h后,采用Trizol法提取细胞总RNA,取500 ng RNA进行逆转录和荧光定量PCR。采用miRNA-221、miRNA-222特异性逆转录引物和PCR引物(由广州锐博公司合成)进行逆转录和PCR(试剂购自广州锐博公司) 检测miRNA-221、miRNA-222的表达,以U6作为内参。采用非特性引物进行逆转录、 SIRT1特异性引物进行PCR(试剂购自上海生工公司)检测 SIRT1,以β-actin作为内参。反应条件参照各自的试剂盒说明书。 SIRT1引物序列为Forwad sequence:5'-CGCA-AGGCGAGCATAGATA-3';Reverse sequence:5'-ACGCTGTGGCAGATTFTT-ATT-3'。
5.免疫印迹法检测SIRT1蛋白的表达量 转染72 h后消化收集细胞,PBS洗2遍,离心,弃上清,每管加入130 μL细胞裂解液,漩涡震荡仪混匀裂解细胞,置于冰上裂解3 min,4 ℃ 12 500× g离心15 min,取上清部分。采用BCA蛋白浓度测定试剂盒(碧云天生物)测定总蛋白浓度。细胞裂解产物与5×loading buffer以体积比4∶1混匀,100 ℃变性8 min,取60 μg总蛋白进行免疫印迹实验。利用ECL显色液和凝胶成像仪(美国Bio-Rad公司)曝光显影,采用Quantity one软件对条带灰度进行量化。
6. miRNA-221和miRNA-222靶基因的预测 利用miRNAanda (http://www.microrna.org/) 对miRNA-221和miRNA-222的靶基因进行预测分析。
三、统计学方法
采用SPSS 17.0软件进行统计分析。所有实验均重复3次以上。数据以x- ±s表示,采用 t检验或One-way ANOVA检验。 P<0.05为差异有统计学意义。
一、pEX-5、miRNA-221 inhibitor和miRNA-222 inhibitor质粒转染效率的观察
以白光下的细胞数为对照,各组转染效率[表达绿色荧光蛋白(GFP)细胞的比例]均>50%,见图1。
二、miRNA-221和miRNA-222对前列腺癌细胞PC-3功能的影响
1.miRNA-221和miRNA-222在细胞中的表达量 转染miRNA-221 inhibitor 和miRNA-222 inhibitor 后,miRNA-221在细胞中的表达下调约50%( t=5.08, P<0.05),miRNA-222下调约75%( t=15.4, P<0.05),见图2。
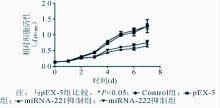
2. miRNA-221和miRNA-222对细胞增殖的影响 利用CCK-8法连续检测7 d各组细胞的细胞活性,绘成生长曲线,见图3。与pEX-5组比较,抑制细胞中miRNA-221和miRNA-222表达后,细胞活性受到抑制。从第3天起,细胞活性抑制效率近1.5倍,差异有统计学意义( t=21.7, P<0.05),随后几天这种差异更为明显。
3. miRNA-221、miRNA-222抑制细胞迁移能力的检测 采用划痕修复实验检测PC-3细胞的迁移能力,见图4。24 h时,Control组、pEX-5组、miRNA-221抑制组、miRNA-221抑制组的闭合率分别为25.0%±2.4%、20.0%±8.6%、9.0%±2.1%、14.0%±11.0%;miRNA-221抑制组、miRNA-221抑制组与pEX-5组之间差异无统计学意义( P>0.05)。48 h时,miRNA-221抑制组、miRNA-221抑制组闭合率分别为25.0%±1.5%、34.0%±2.9%,与pEX-5组(57.0%±7.0%)比较,差异均有统计学意义( t值分别为7.5、3.4, P<0.05)。72 h时,miRNA-221抑制组、miRNA-221抑制组闭合率分别为47.0%±2.7%、59.0%±9.9%,与pEX-5组(100.0%±1.0%)比较差异均有统计学意义( t=15, P<0.01; t=6.8, P<0.05)。见表1。
| 表1 Control组、pEX-5组、miRNA-221抑制组和miRNA-222抑制组细胞的迁移率(闭合率)(%) |
4. miRNA-221、miRNA-222靶基因的预测 利用miRNAanda (http://www.microrna.org) 对miRNA-221和miRNA-222靶基因进行预测分析,结果显示miRNA-221和miRNA-222与 SIRT1存在靶向结合位点。见图5。
5.转染miRNA-221 inhibitor和miRNA-222 inhibitor后SIRT1蛋白表达水平的变化 PC-3细胞转染pEX-5、miRNA-221 inhibitor和miRNA-222 inhibitor后,miRNA-221抑制组、miRNA-221抑制组SIRT1蛋白相对表达量分别为0.26±0.021、0.21±0.005 8,与pEX-5组(0.14±0.017)比较差异均有统计学意义( t值分别为6.3、5.3, P均<0.05)。见图6。
6. 转染miRNA-221 inhibitor和miRNA-222 inhibitor后 SIRT1 mRNA表达水平的变化 PC-3细胞转染pEX-5、miRNA-221 inhibitor和miRNA-222 inhibitor后,pEX-5组、miRNA-221抑制组和miRNA-222抑制组 SIRT1 mRNA的相对表达量分别为0.99±0.05、1.2±0.1、1.1±0.05,各组间差异均无统计学意义( F=3.6, P>0.05),见图7。
miRNA是近几年发现的一种非编码短链RNA,在多种肿瘤的发生发展过程中发挥癌基因和抑癌基因的作用[ 9, 10, 11]。miRNA根据碱基互补配对原则,靶向结合mRNA,通过降解靶mRNA或者抑制其翻译过程从而调节靶基因的表达[ 12]。
miRNA-221、miRNA-222定位于Xp11.3[ 13],在多种肿瘤(包括前列腺癌)中表达上调[ 14, 15, 16, 17]。研究已证实,miRNA-221和miRNA-222可通过靶向调节 p27 kip1、 PTEN、 MMP1、 PUMA等多种基因的表达,影响肿瘤的发生、发展过程,包括细胞增殖、凋亡、迁移和侵袭等[ 16, 17, 18, 19, 20]。SIRT1为NAD+依赖性的组蛋白去乙酰化酶,参与多种生理过程的调节,但对不同类型癌症发展的作用千差万别,可发挥抑癌基因[ 21, 22, 23, 24]和癌基因[ 25, 26, 27, 28, 29, 30, 31]的双重作用。Wang等[ 8]证实SIRT1在前列腺癌中表达下调,发挥抑癌基因的作用。
本研究通过抑制miRNA-221和miRNA-222的表达,发现前列腺癌细胞系PC-3细胞的增殖、迁移能力显著降低。为了研究miRNA-221、miRNA-222在前列腺癌细胞中影响增殖和迁移的机制,本研究观察了抑制miRNA-221、miRNA-222表达后SIRT1蛋白表达的变化,发现SIRT1蛋白表达明显上升,但在基因水平没有明显的差异。miRNA调节靶基因的途径主要有两个:一方面通过完全互补结合靶基因导致靶基因的降解而影响其表达;另一方面通过不完全互补配对结合,抑制靶基因的翻译过程而达到抑制效果[ 32]。从本研究结果分析,miRNA-221和miRNA-222可能在翻译水平影响了SIRT1的表达,而非基因水平。另外,通过miRNA靶标预测软件miRNAanda(http://www.microrna.org/microrna/home.do)分析,miRNA-221、miRNA-222与SIRT1存在靶向结合关系。至于miRNA-221和miRNA-222是否与SIRT1存在直接靶向结合作用,有待于在后续实验中进行验证。
综上所述,miRNA-221和miRNA-222可以影响PC-3细胞的增殖和迁移以及SIRT1的表达,但对SIRT1表达的影响是否通过直接靶向抑制来调节的尚待验证。
(收稿日期:2013-12-06)
(本文编辑:龚晓霖)
| [1] |
|
| [2] |
|
| [3] |
|
| [4] |
|
| [5] |
|
| [6] |
|
| [7] |
|
| [8] |
|
| [9] |
|
| [10] |
|
| [11] |
|
| [12] |
|
| [13] |
|
| [14] |
|
| [15] |
|
| [16] |
|
| [17] |
|
| [18] |
|
| [19] |
|
| [20] |
|
| [21] |
|
| [22] |
|
| [23] |
|
| [24] |
|
| [25] |
|
| [26] |
|
| [27] |
|
| [28] |
|
| [29] |
|
| [30] |
|
| [31] |
|
| [32] |
|